
COVID-19 vaccine based on new technology tested in clinical study
![SARS-CoV-2 RBD-specific antibodies Vertical lines indicate the first and second ABNCoV2 vaccination (28 days after first vaccination). (A) Concentration of RBD-specific antibodies of groups 1 to 3 (6 μg, 12 μg, and 25 μg ABNCoV2 non-adjuvanted [groups labelled A] and MF59-adjuvanted [groups labelled B]) up to day 42 after the first vaccination (14 days after the second vaccination). (B) Concentration of RBD-specific antibodies of groups 1A, 2A, 3A, 4, and 5 (6 μg, 12 μg, 25 μg, 50 μg, and 70 μg) 14 days after second vaccination. (C) Concentration of RBD-specific antibodies of groups receiving the optimal doses 25 μg and 50 μg ABNCoV2 until day 42 after the first vaccination. (D) Concentration of RBD-specific antibodies of groups receiving the optimal doses 25 μg and 50 μg ABNCoV2 until day 196 after the first vaccination (end-of-study visit). Different colors indicate types of licensed SARS-CoV-2 vaccines that participants received during the follow-up period. RBD=receptor binding domain. Credit: The Lancet Microbe (2023). DOI: 10.1016/S2666-5247(22)00337-8 COVID-19 vaccine based on new technology tested in clinical study](https://scx1.b-cdn.net/csz/news/800a/2023/covid-19-vaccine-based.jpg)
A new COVID-19 vaccine dependent on a various platform than present-day vaccines on the sector has been analyzed in human beings for the initial time by scientists at Radboud university professional medical center. Administration of this vaccine in wholesome examine contributors was nicely tolerated and led to a superior immune response. The efficiency of the vaccine is currently currently being more investigated. To start with success are envisioned later this yr.
The new vaccine, named ABNCoV2, is unique from coronavirus vaccines promoted to day: the mRNA vaccines (such as these from Pfizer and Moderna), the vector vaccines (this kind of as these from Janssen and AstraZeneca) and the protein vaccine created by Novavax.
ABNCoV2 is a capsid-like virus-like particle (VLP) vaccine. This means that the new vaccine consists of aspects that resemble virus particles. To the immune process, these particles look like a virus, but they are not able to replicate. The virus-like particles can be billed with antigens such as the spike protein of the coronavirus SARS-CoV-2. As a end result, the body swiftly reacts to the virus by making antibodies and T cells.
This vaccine was developed by the Danish biotechnology organization AdaptVac, in collaboration with Radboudumc and the Stop-nCoV consortium. Radboudumc was dependable for the style and design and implementation of the analyze.
Number of aspect effects
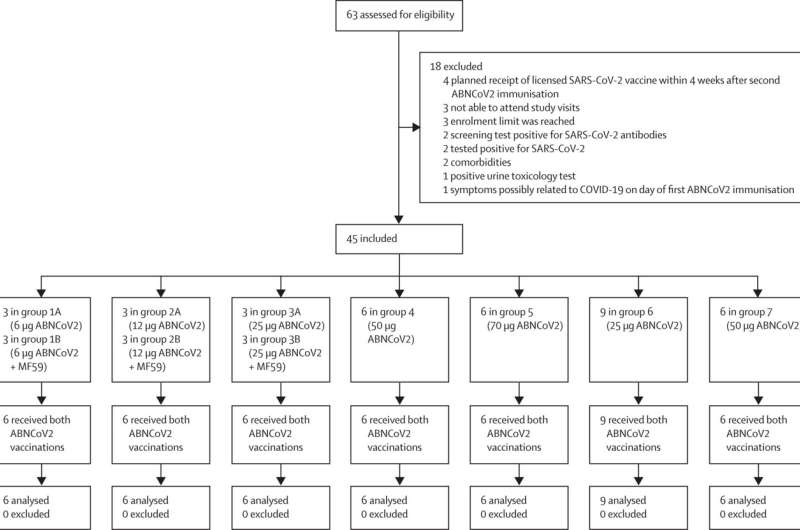
This research, conducted by a team of scientific scientists at Radboud college health-related middle, investigated security and tolerability of the vaccine. 45 nutritious analyze individuals, who had not still experienced COVID-19 and had not been vaccinated, been given two doses of the new vaccine. They have been followed for 6 months following the 2nd vaccination. The members manufactured antibodies and T cells versus SARS-CoV-2.
Additionally, the research showed that the vaccine was nicely tolerated by the examine members: a few side effects ended up described. Principal investigator Dr. Benjamin Mordmüller, professor of medical microbiology at Radboud college professional medical middle, suggests, “The vaccine has exceeded our anticipations in terms of immunity and tolerability.” The final results have now been published in The Lancet Microbe.
Usefulness
To determine the efficiency of this vaccine, stick to-up exploration is needed. Various efficacy reports are at the moment getting spot. Medical investigator Merel Smit is hopeful about this: a vaccine primarily based on a equivalent principle is the vaccine against the HPV virus that can induce cervical most cancers, among the other items.
With this HPV vaccine, immunity is preserved for a very long period of time and no booster later on in lifestyle is needed. If this also applies to the new vaccine, it may well necessarily mean that any booster vaccinations can be provided at longer intervals.
Other infectious disorders
An significant advantage of this variety of vaccine is its ability to be swiftly adapted in the party that the SARS-CoV-2 virus acquires mutations that reduce the efficacy of the ABNCoV2 vaccine. In addition, this so-referred to as cVLP system, the foundation of the vaccine, is highly flexible and can also be utilised to create enhanced vaccines for international infectious conditions, this kind of as malaria and influenza. This was not yet attainable with the vaccine versus the HPV virus. A malaria vaccine is presently remaining formulated based mostly on this vaccine, which is predicted to be tested subsequent calendar year.
“The outcomes are incredibly fantastic information for the improvement of vaccines towards a extensive assortment of infectious health conditions for which we have no or only partially energetic standard vaccines,” states Mordmüller.
The study is posted in The Lancet Microbe.
More information and facts:
Merel J Smit et al, Initial-in-human use of a modular capsid virus-like vaccine platform: an open-label, non-randomised, section 1 scientific trial of the SARS-CoV-2 vaccine ABNCoV2, The Lancet Microbe (2023). DOI: 10.1016/S2666-5247(22)00337-8
Citation:
COVID-19 vaccine based mostly on new know-how tested in clinical review (2023, January 25)
retrieved 26 January 2023
from https://medicalxpress.com/information/2023-01-covid-vaccine-centered-technological know-how-clinical.html
This document is issue to copyright. Apart from any truthful dealing for the reason of non-public review or investigate, no
part may perhaps be reproduced without the published authorization. The content is delivered for data uses only.
